Understanding the Solid Nature of Atoms: A Quantum Perspective
Written on
Chapter 1: The Mystery of Solid Objects
Have you ever questioned why, despite atoms being composed of approximately 99.9% empty space, objects appear solid and tangible? This article aims to shed light on this intriguing phenomenon. It turns out that much of what we learn in school is a simplified version of reality. I will clarify why we perceive objects as solid by examining the behavior of electrons.
Topics explored in this article:
- Electron Orbitals
- The Concept of Electron Dance
- Understanding the Sensation of Solidity
Electron Orbitals
The movement of electrons around the nucleus is far more complex than the simplistic model we learned in our early education. Electrons do not orbit the nucleus like planets orbit the sun. Instead, we can visualize electrons as a cloud or a flock of birds, where their individual movements are too rapid and unpredictable to track precisely. However, we can discern the overall shape of this "cloud," which represents the probability of finding an electron in a specific location, termed orbitals. Each electron in an atom has a unique orbital shape, as depicted in the following image.
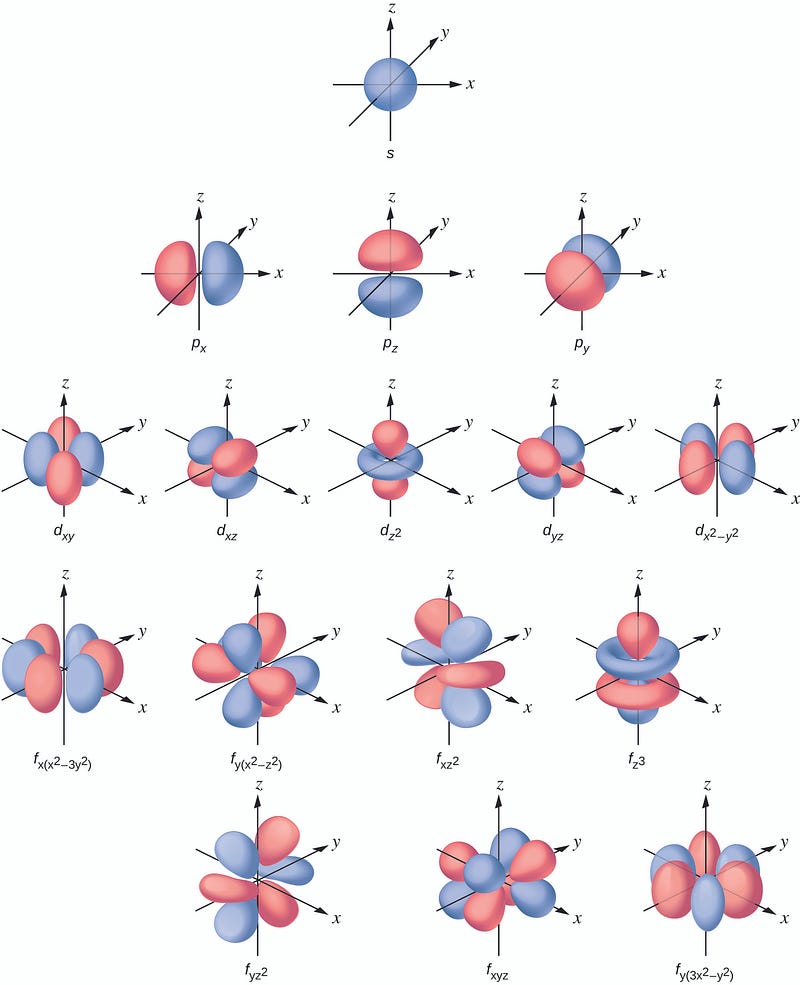
Understanding Electron Dance
To aptly describe the behavior of electrons in their orbitals, the term 'Electron Dance' is particularly fitting. This motion is not random; it adheres to specific patterns as outlined by Erwin Schrödinger. Depending on the electron's energy level, these patterns fluctuate. The following image illustrates the various probability clouds or electron dance patterns at different energy levels, specifically for the Hydrogen atom. Electrons with higher energy levels exhibit a more rapid dance, whereas those with lower energy levels display a different pattern.
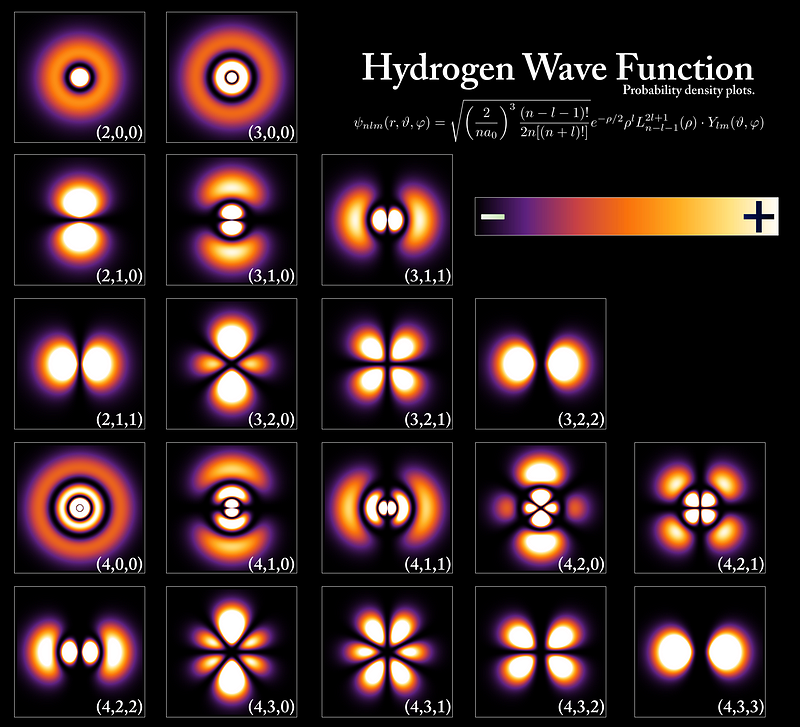
The first video, "Electron Dance Status Report: Oct 2019," provides a visual representation of electron dynamics, enhancing our understanding of their behavior.
When electrons absorb energy, they transition to higher energy states, adopting a more vigorous dance pattern. Conversely, a loss of energy results in a slower dance. This shifting state occurs quickly; for example, when photons hit the electrons, they absorb the energy and elevate to a higher, albeit unstable, state. Consequently, when light interacts with solid objects, the electrons absorb photons, preventing light from passing through, thus creating the perception of solidity.
Why Do We Perceive Solidity When Touched?
While we understand why objects appear solid visually, the question remains: why do we perceive them as solid when we make physical contact? One might think that the repulsion between negatively charged electrons in our hands and those in the object causes this sensation. However, this explanation is not entirely accurate, as noted by Prof. Roger Barlow. The solid sensation derives from the interactions of the dancing electrons.
When we touch an object like a table, the electrons in our hands come close to those in the table. The proximity to the nucleus of the table's electrons alters their dance pattern. Since electrons occupy specific energy levels, they cannot perform the same dance simultaneously. Therefore, the electrons from our hand must move to higher orbitals, a process that requires energy. This energy is provided by the force exerted when we press our hand against the object.
The greater the force applied, the more energy is supplied, allowing more electrons to occupy higher energy states. Attempting to push all the atoms from our hand through the table demands an immense amount of energy—far beyond what our muscles can generate. This resistance is what prevents us from moving through solid objects, despite the fact that atoms are primarily empty space.
Chapter 2: Further Insights
The second video, "Electron Dance (YAS185) by Mark Revell," delves deeper into the concept of electron dynamics, enriching our understanding of this complex subject.
Contact
For more insights into Quantum Computing and Machine Learning, follow me on Medium. Also, check out my GitHub and LinkedIn.