Innovative Aluminum Devices: The Future of Medical Technology
Written on
Chapter 1: Breakthrough in Drug-Delivery Devices
Recent advancements in medical technology have led to the development of innovative drug-delivery systems that can safely dissolve within the body.
This paragraph will result in an indented block of text, typically used for quoting other text.
Section 1.1: The Challenge of Device Removal
Metal stents and similar biomedical devices have been utilized in healthcare for many years. A significant issue with these devices is the challenge of their removal after they have served their purpose. While most existing devices are constructed from polymers, researchers are now investigating the potential of using more resilient metals.
Subsection 1.1.1: MIT's Innovative Approach
A team of researchers at MIT has made considerable progress in this area. Previously, they focused on creating ingestible devices capable of remaining in the digestive system for extended periods, releasing medication at designated intervals. Their latest experiments involve substituting polymers with metals, while also demonstrating a method for the safe removal of these devices once they are no longer needed.

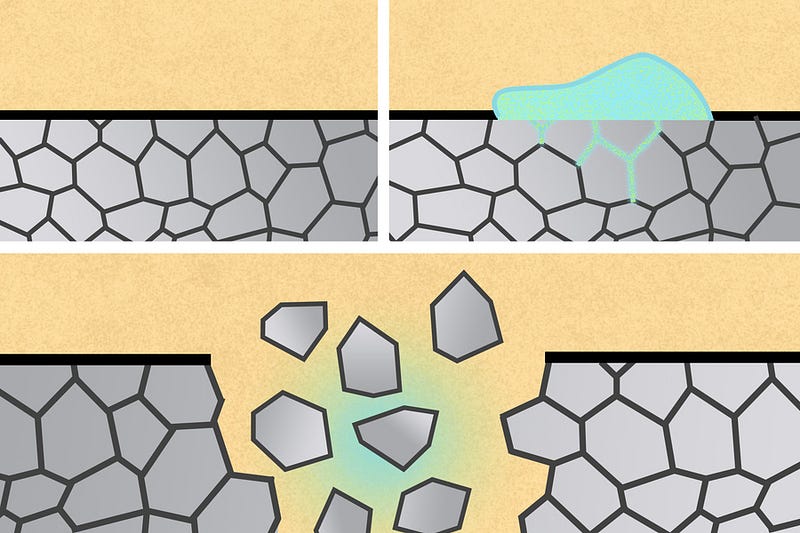
The newly designed biomedical devices, which can serve as stents, staples, or drug reservoirs, are made from aluminum. These devices can be easily disintegrated by exposing them to a liquid metal known as eutectic gallium-indium (EGaIn). This process occurs as the liquid metal infiltrates the grain boundaries of the solid metal, which are the interfaces between its tiny crystalline structures.

Section 1.2: Implications of Liquid Metal Embrittlement
“It’s a really dramatic phenomenon that can be applied to several settings. What this enables, potentially, is the ability to have systems that don’t require an intervention such as an endoscopy or surgical procedure for removal of devices.”
~ Prof. Giovanni Traverso, Lead Researcher
Inspiration for this research stems from the process of liquid metal embrittlement, which is well-documented and leads to the failure of metal structures made from zinc and stainless steel. The research team has sought to leverage this failure mechanism in a constructive manner.
Chapter 2: Practical Applications and Future Directions
The researchers began by using eutectic gallium-indium as the liquid metal and aluminum for the medical devices, capitalizing on the fact that aluminum is prone to embrittlement when exposed to gallium. This exposure prevents aluminum from forming a protective oxide layer, increasing its vulnerability to degradation when in contact with water.

MIT engineers have demonstrated that aluminum-based medical devices can be disintegrated within the body when exposed to gallium-indium. The liquid metal infiltrates the grain boundaries of the aluminum, causing rapid disintegration. The research indicates that nanoparticles and microparticles of gallium-indium suspended in fluid can achieve similar results.
For gastrointestinal applications, the team tested a star-shaped device with arms attached to a central elastomer via a hollow aluminum tube. In animal trials, aluminum staples used to hold tissue together were successfully dissolved with a coating of gallium-indium. Furthermore, an aluminum stent implanted in esophageal tissue was also found to break down using this method.
The current esophageal stents are typically made from a nickel-titanium alloy known as nitinol, and the research team's next objective is to develop dissolvable devices from this material. The complete study has been published in the Journal of Advanced Materials.
The first video titled "Designing Medical Devices: Getting Started" dives into the foundational aspects of medical device design, providing insights on the crucial steps involved.
The second video, "Webinar: Medical Devices in the Home: Design Considerations and FDA Guidance," discusses the important factors to consider when designing medical devices for home use, including regulatory challenges.