T Cell Receptors: Key Players in Adaptive Immunity
Written on
Chapter 1: Introduction to T Cell Receptors
T cell receptors (TCRs) are essential components of the adaptive immune system. They play a pivotal role in the activation of T cells, which are crucial for identifying and responding to pathogens. Each TCR is comprised of two proteins that form the antigen-recognizing segment of the receptor. These proteins can be either an α paired with a β subunit or a γ paired with a δ subunit. This pairing determines whether the TCR is classified as αβ or γδ. The majority of T cells in the body possess αβ TCRs, while only a small fraction—less than 10%—have γδ TCRs. TCRs are transmembrane proteins that engage with antigens presented by target cells.
For most TCRs, the antigens recognized are peptide fragments of proteins. These peptides are displayed on major histocompatibility complexes (MHCs). MHC molecules feature a subunit known as β2-microglobulin (β2m) and proteins from the human leukocyte antigen (HLA) family. The HLA proteins bind to the antigen, and β2m is crucial for the receptor's transportation to the cell surface.
TCRs specifically bind to peptides presented by MHC molecules. The genes that encode TCR subunits and HLA proteins exhibit considerable variability. This genetic variability, including complex rearrangements, enhances the diversity of an individual's immune response, enabling recognition of both self and non-self entities. The polymorphism of HLA and the complexity of TCR genes contribute to the adaptive immune response tailored to specific antigens from pathogens.
Some TCRs also have the capability to recognize lipids. These lipids are displayed on protein complexes that include β2m and CD1 proteins rather than HLA proteins. Additionally, certain TCRs can identify metabolites produced by microbial activity, displayed on complexes with β2m and MR1 proteins.
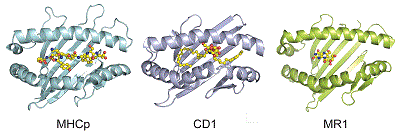
The structures of MHC, CD1, and MR1 proteins that present antigens to TCRs share similarities, despite their binding to different types of molecules—peptides for MHC, lipids for CD1, and metabolites for MR1.
Chapter 2: TCR Specificity and Antigen Recognition
TCRs are specific to their corresponding antigens. A TCR that binds to a peptide presented by an MHC molecule will not recognize lipids presented by CD1 or metabolites displayed by MR1.
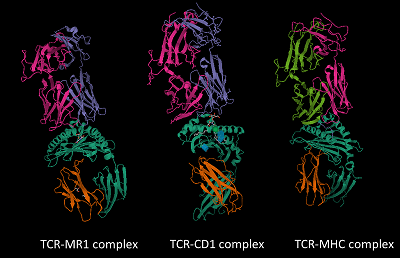
The top structures represent the TCR's α and β subunits, while the bottom structures signify the antigen-presenting proteins with MHC, CD1, and MR1. The recognition of antigens occurs at the interface between the TCR and the antigen-bound proteins.
Each TCR that recognizes an MHC-presented peptide is also tailored to that specific MHC molecule. Unlike the HLA-encoding genes, the genes for MR1 and CD1 are relatively constant among individuals. This uniformity means that TCRs across the population are capable of recognizing MR1- or CD1-presented antigens. In contrast, TCRs recognizing MHC-presented antigens are unique to each person.
The TCRs target non-self molecules from pathogens, while typically avoiding recognition of self molecules. This specificity is vital, as T cells that mistakenly identify self molecules can lead to autoimmune diseases. This challenge is particularly relevant in cancer, where tumor cells may not be entirely foreign to the immune system, complicating the immune response.
Chapter 3: T Cell Response to Cancer
This video, "Lecture 4c: T Cell Signaling + Activation," provides insights into the signaling pathways involved in T cell activation and how these processes are crucial for immune responses.
The video titled "Immunology: T cell receptor structure and function" explores the architecture and functionality of TCRs in detail, enhancing our understanding of their role in immune responses.