Exploring the Revolutionary LF Chip in Immune System Research
Written on
Chapter 1: Unraveling Immune Responses
The ongoing pandemic has highlighted the intricacies of our immune system. Variations in individual reactions to COVID-19 infections have emphasized the gaps in our scientific understanding of this critical biological defense mechanism. While some individuals faced severe symptoms requiring hospitalization, others displayed only mild responses, and a few experienced multiple infections, while many remained unaffected. This variability underscores the need for deeper insights into immune function.
Researchers at the Wyss Institute for Biologically Inspired Engineering at Harvard University have developed a microfluidic tool known as the LF Chip, designed to decode the complexities of the immune system. This innovative device allows scientists to cultivate human B and T cells, prompting them to create functional lymphoid follicles—structures essential for immune responses located in lymph nodes and other areas of the body.
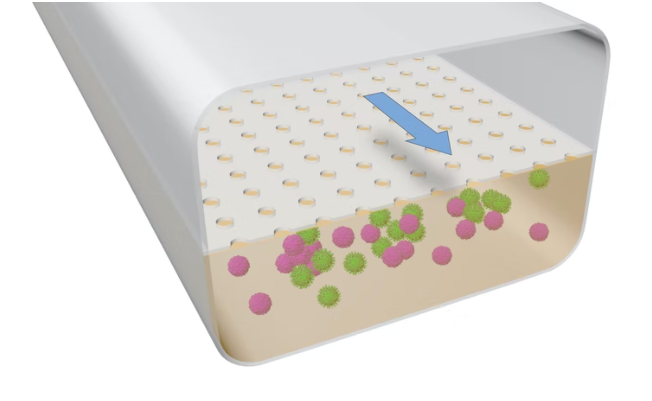
These lymphoid follicle (LF) Chips feature two interconnected chambers, where B cells and T cells are cultivated in a lower channel lined with an extracellular matrix (ECM). The upper channel continuously supplies nutrients to the cells below, resulting in a complete immune response when exposed to a specific antigen.

"Our LF Chip provides a method to simulate the intricate dynamics of human immune responses to infections and vaccinations, potentially accelerating vaccine development significantly."
~ Girija Goyal, First Author

In addition to enhancing our comprehension of immune responses, the LF chips may predict vaccine efficacy, thus aiding in the selection of the most effective candidates. This advancement could replace current trial methods that rely on ethically questionable practices involving primates, saving considerable time in the process.
Researchers noted that the ethical concerns surrounding animal testing are compounded by the significant differences between animal and human immune systems, which can lead to inaccurate predictions of human responses. Interestingly, the development of the LF chip was serendipitous; the scientists initially aimed to investigate how circulating B and T cells modify their behavior upon entering tissues.
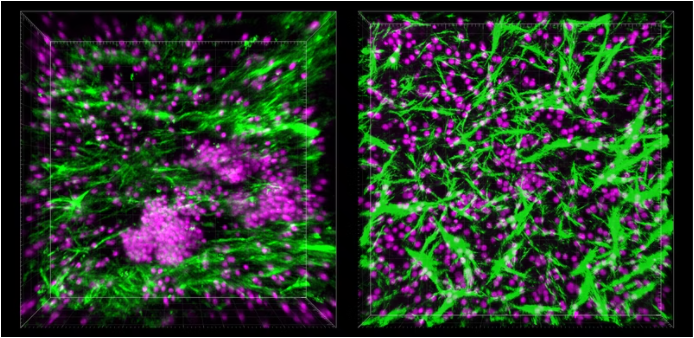
Upon culturing B and T cells in the LF Chip under flowing conditions, they naturally formed three-dimensional structures identified as nascent lymphoid follicles. In contrast, no such formations occurred when the cells were maintained in static conditions. The realization that these structures were capable of secreting CXCL13—a marker indicative of lymphoid follicle formation during chronic inflammation—was pivotal. Furthermore, researchers discovered activation-induced cytidine deaminase (AID) produced by B cells, essential for targeted responses to specific antigens.

"The LF Chip provides a cost-effective, rapid, and predictive framework for exploring human immune responses to infections and vaccines, which we hope will enhance vaccine development for various diseases in the future."
~ Donald Ingber, Corresponding Author

The absence of CXCL13 and AID in cells grown in traditional two-dimensional dishes prompted the team to conclude that they had successfully created functional lymphoid follicles from circulating blood cells. Subsequent experiments focused on investigating the human immune system’s reactions to vaccines using these functional structures. In their initial trial, researchers administered a vaccine against the H5N1 strain of influenza alongside an adjuvant known as SWE, which enhances immune responses to vaccines.
As reported, the LF Chips that received both the vaccine and the adjuvant generated significantly higher levels of plasma cells and anti-influenza antibodies compared to those grown in 2D cultures or those that received the vaccine alone. This experiment was replicated with cells from eight different donors using the Fluzone influenza vaccine, confirming a similar outcome with notable production of plasma cells and antibodies in the LF Chips.
With the promising results from these initial trials, the research team is actively collaborating with pharmaceutical companies and the Gates Foundation to test various vaccines and adjuvants using their LF Chips. The researchers assert that LF Chips offer a more efficient, cost-effective, and predictive model for studying human immune responses to both infections and vaccines. Their findings are published in the Journal of Advanced Science and supported by DARPA.
This video explains the innate and adaptive defenses of the immune system, illustrating how these systems work together to protect the body.
This video details the elements of the immune system and their specific roles in defending against pathogens, providing an updated overview of current research.